biofilm formation protocol
Two methods were used to measure biofilm formation. Add 200 μL of 30 acetic acid to all wells that were stained to solubilize the crystal.

Microtiter Dish Biofilm Formation Assay Protocol
These changes are reflected in t.

. Films were fixed by incubating the plates at 60C for 1 hour. The extent of biofilm formation was determined by applying three different formulas. It is clear that microorganisms undergo profound changes during their transition from planktonic free-swimming organisms to cells that are part of a complex surface-attached community.
The presence of an orthopedic implant reduces the bacterial concentration required to induce infection by 100000 times 2 since bacteria can. 33 CV-Stained Biofilm Quantitation 1. US5928889A - Protocol for simulated natural biofilm formation - Google Patents This invention provides a methodology for controlled biofilm formation in accordance with a.
Ii bf abcw. Pipette up and down to assure that the stained biofilm is well solubilized and then transfer 100 μL of each sample to a new 96-well optically clear flat-bottom plate. The monitor protocol in the annular reactor subjects the bacterial consortia of the simulated natural biofilm to simulated.
8 5 The first colonist bacteria of a biofilm may adhere to the surface initially by the weak van der Waals forces and hydrophobic effects. This output reflects only the authors view and the Research Executive Agency REA cannot be. Biofilm formation was evaluated by adding 200 µL of 30 acetic acid to each well after staining with 50 L of a 01 wv crystal violet solution and then measuring the OD 600 of the eluate.
Nature Protocols - A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing Skip to main content Thank you. This invention provides a methodology for controlled biofilm formation in accordance with a monitored protocol. Biofilm formation is a strategy by which microorganisms survive and adapt to the involving environment particularly adverse conditions.
Violet remaining is bound to a biofilm at the bottom of a well. The development of a biofilm includes attachment of cells to a surface multiplication maturation and production of a polymeric matrix and finally microbial detachment and colonization of new surfaces. Rings of crystal violet around a well are not indicative of biofilm formation and should be rinsed again as excess stain will skew the results of the assay.
In a typical embodiment a natural biofilm inoculum is used to form a simulated natural biofilm on retrievable slides in an annular reactor. This will solubilize the CV. Crystal Violet 1 CV1.
Bacteria adhesion and biofilm formation differ between biomaterial surfaces used in orthopedic implants. Step 1 - Lysis breakdown and Detachment of the Polysaccharide Matrix eg Biofilm Take remedy on an empty stomach 30 to 45 minutes before food minimally 30 to 60 minutes before Step 2 Interfase Plus Klaire Labs 2 to 4 capsules before or in-between meals 15 to 30 minutes prior or 90 minutes after meals. The formation of a biofilm begins with the attachment of free-floating microorganisms to a surface.
Protocols for the study of biofilm formation in a microfluidic device that mimics porous media are discussed. Significantly less biofilm is formed on ceramic surfaces compared to polyethylene and metal. Pipette 200 μL of 30 acetic acid solution into each well.
Biofilm formation as microbial development Biofilms can be defined as communities of microorganisms attached to a surface. Incubate for 1015 min. The microfluidic device consists of an array of micro-pillars and biofilm formation by Pseudomonas fluorescens in this device is investigated.
Leave the plate face up on the bench top overnight to dry. The protocol I follow utilizes multichannel pipettes opposed to other rinsing and removal methods I have read about others using. National Center for Biotechnology Information.
Protocols for analysis of biofilm formation in water and soil substrates. Eluates with an optical density 25 were diluted 110. From 1100 diluted overnight broth plates are inoculated and.
I bf ab cw where bf is the biofilm formation ab is the od 540nm of stained attached bacteria and cw is the od 540nm of stained control wells containing bacteria-free medium only unspecific or abiotic factors kadurugamuwa et al. This project has received funding from the European Unions Horizon 2020 research and innovation programmeunder grant agreement No.

Biofilm Formation Assay Kit Dojindo

An Improved Crystal Violet Assay For Biofilm Quantification In 96 Well Microtitre Plate Biorxiv

Biofilm Formation Assay Kit Testpiece Dojindo Eu

Biofilm Eradication Testing For Antimicrobial Efficacy
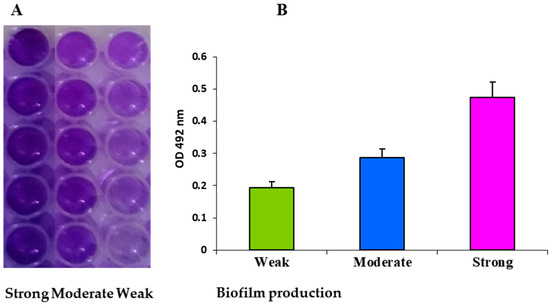
Ijms Free Full Text Expression Of The Biofilm Associated Genes In Methicillin Resistant Staphylococcus Aureus In Biofilm And Planktonic Conditions Html

Biofilm Formation Assay In Pseudomonas Syringae Bio Protocol

Crystal Violet Assay To Assess The Antibiofilm Activity Of Samples Download Scientific Diagram

Schematic Representation Of The Steps Involved In The Protocol For Download Scientific Diagram

Polystyrene Microtiter Plate Biofilm Assay Of A Pleuropneumoniae Download Scientific Diagram

Biofilm Formation Assay Kit Dojindo

Pdf Different Methods For Culturing Biofilms In Vitro

Optimized Protocol For H Volcanii Immersed Liquid Biofilm Formation Download Scientific Diagram

Biofilm Formation Assay In Pseudomonas Syringae Bio Protocol

Biofilm Formation Assay Kit Dojindo

Schematic Crystal Violet Assay On Biofilms In A Microtiter Plate Download Scientific Diagram

An Improved Crystal Violet Assay For Biofilm Quantification In 96 Well Microtitre Plate Biorxiv

Biofilm Eradication Testing For Antimicrobial Efficacy
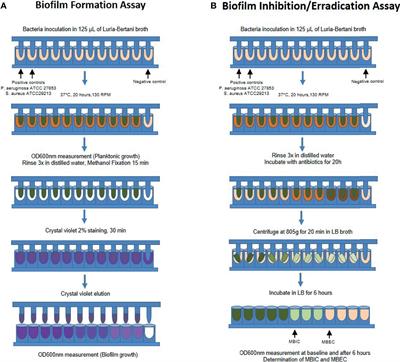
Frontiers Biofilm And Planktonic Antibiotic Resistance In Patients With Acute Exacerbation Of Chronic Rhinosinusitis
Comments
Post a Comment